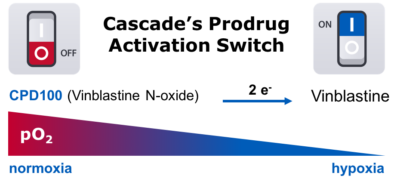
Cascade Prodrug employs a platform technology that has led to our lead candidate, CPD100Li. CPD100Li is a liposomal formulated proprietary N-oxide prodrug of vinblastine, an approved cytotoxic vinca-alkaloid that has been used clinically in solid tumor and hematologic malignancies. CPD100Li is stable at normal tissue O2 levels but ‘switches’ to cytotoxic vinblastine under hypoxic conditions. By creating a novel on-off switch for vinblastine, we take advantage of tumor hypoxia and trigger drug conversion in the tumor where it’s needed, thus limiting the exposure of healthy tissue to a potent cytotoxic agent. Cascade Prodrug believes that this technology will significantly increase the maximum-tolerated-dose of chemotherapy, thereby improving outcomes in patients with solid tumors.
Cascade Prodrug’s platform brings three key therapeutic enhancements that will provide patients with improved treatment options for treating a variety of tumor types: efficacy, safety and enhanced tumor penetration. These therapeutic enhancements are made possible by our proprietary hypoxia-activated prodrug switch. CPD100Li has potent anti-tumor activity when exposed to the tumor microenvironment; it effectively masks vinblastine toxicity until it reaches the tumor, so it is much safer for the patient; encapsulation into small liposomal particles enables extended circulation times and enhanced tumor penetration.
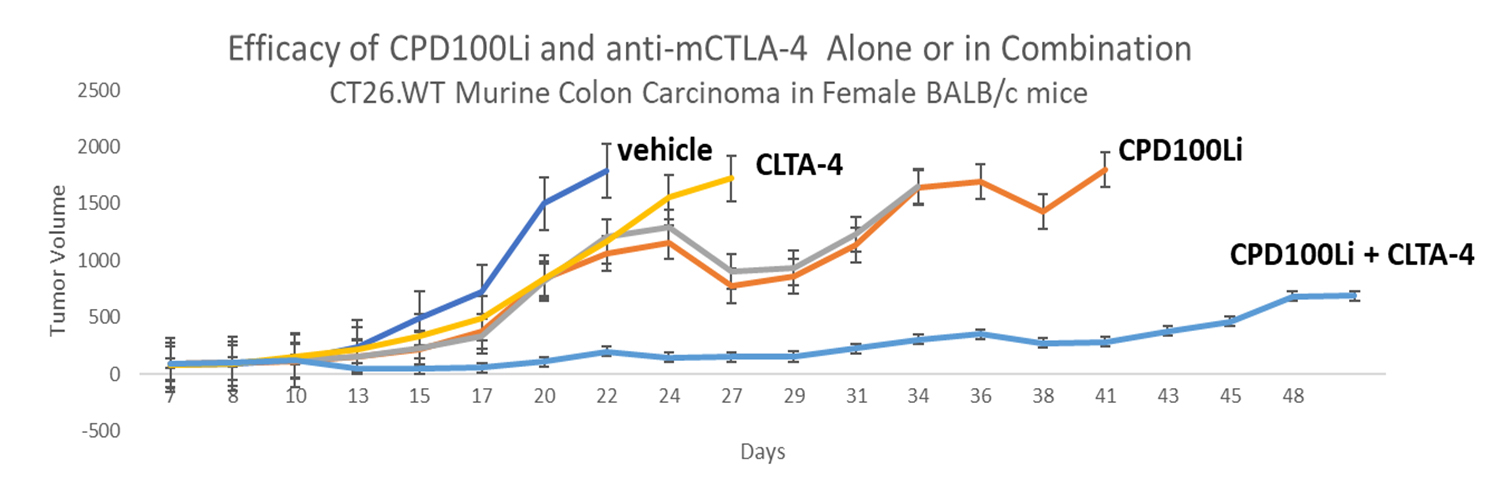
Furthermore, CPD100Li has shown a reduction in resident and infiltrating immunosuppressive lymphocytes, with demonstrable preservation of anti-tumor macrophages and marked reduction in tumor-friendly macrophages and strong synergy when combined with an anti CTLA-4 agent in a mouse colon cancer model. This represents another potential development pathway, i.e. exploring combination therapy with immune modifying agents.
EFFICACY | SAFETY | PENETRATION
Potent Anti-Tumor Efficacy
CPD100Li exhibits potent anti-tumor efficacy in established preclinical xenograft models in which hypoxic tumors develop. In the A549 non-small cell lung cancer model, CPD100Li significantly outperformed both cisplatin (treatment control) and unformulated CPD100. CPD100Li is highly effective in the Panc-1 orthotopic pancreatic cancer model, a fast-growing tumor model that is normally resistant to treatment. CPD100Li also showed strong synergy in combination with an anti-CTLA-4 agent in a CT26 colorectal model.
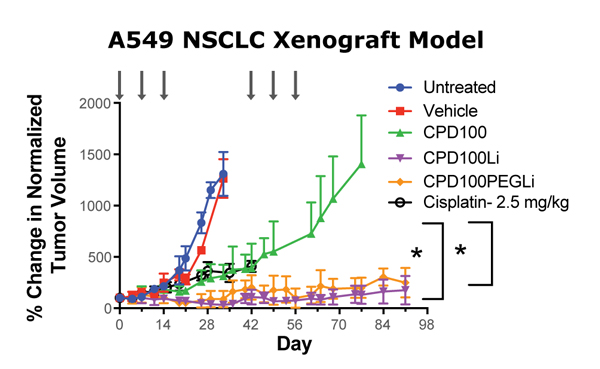
Non-small cell lung cancer solid tumor xenograft
CPD100 dosed at 40 mg/kg (iv) in all on-treatment cohorts
Cisplatin dosed at 2.5 mg/kg
tumor volumes at start of treatment normalized to 100%
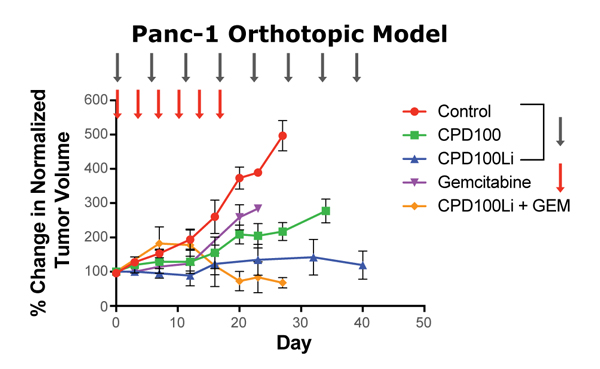
Highly aggressive; difficult to treat; extreme hypoxia
CPD100 dosed at 40 mg/kg (iv) in all on-treatment cohorts
Gemcitabine dosed at 100 mg/kg (ip)
tumor volumes at start of treatment normalized to 100%
Summary
- Liposomal formulation enhances activity of unformulated CPD100 in multiple models
- Activity demonstrated with CPD100Li monotherapy
- Strong synergy shown in combination with gemcitabine and an anti-CTLA-4 agent in Panc-1 model and CT26 colorectal model respectively
Improved Safety Profile
We believe CPD100Li’s hypoxia targeted, liposomal formulated profile will offer patients a significantly improved safety profile relative to currently approved chemotherapy agents. CPD100Li has the potential to significantly reduce the side effects that would accompany an equivalent amount of vinblastine thereby improving outcomes in patients with solid tumors.
- CPD100Li has been well tolerated in all in vivo studies conducted to date in mice
- No acute toxicities observed to date (MTD = 40 mg/kg)
- No weight loss in tumor-bearing mice on treatment
- Modest elevations of liver enzymes and hematological markers
- Unformulated CPD100 has >10X greater MTD than vinblastine
Enhanced Penetration of Solid Tumors
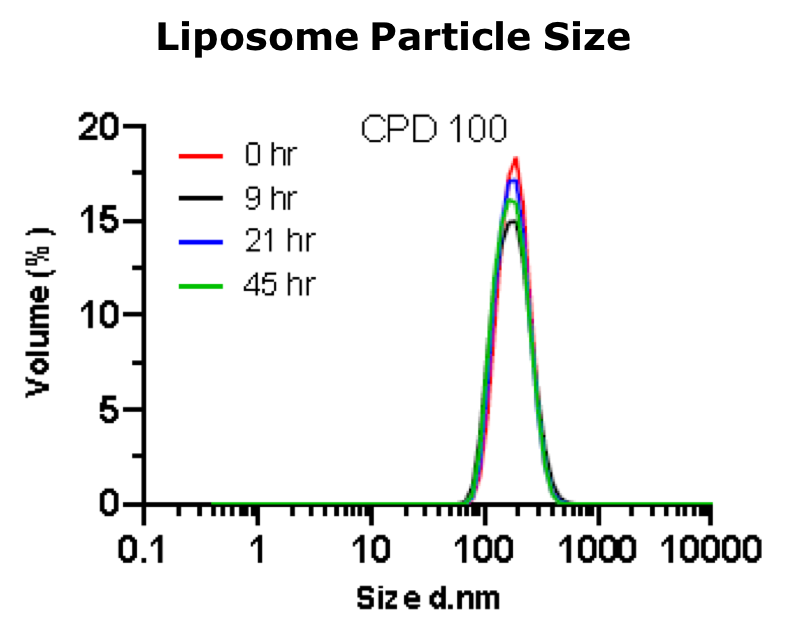
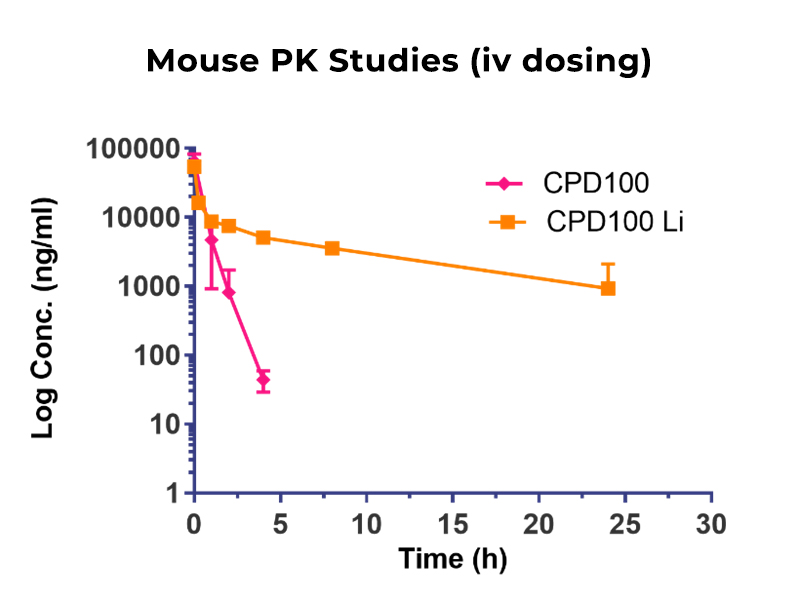
CPD100Li has been engineered to enhance tumor penetration with an average particle size of 170 nm, which allows tumor penetration through the aberrant vascular endothelium found in many tumor types. Liposomal formulation also extends the circulation half-life of CPD100 by more than 10-fold. In mouse PK studies, unformulated CPD100 is rapidly cleared with a t1/2 of 0.4 h. By contrast, the t1/2 clearance rate observed with CPD100-Li was 5.5 hrs. The small particle size, combined with extended circulation time, optimize the potential for efficient tumor penetration.